Introduction
Although Doppler assessment of the fetal branch pulmonary arteries is not part of routine fetal echocardiography, it has a few specific and essential indications. Examining blood flow beyond the main pulmonary artery provides insight into the functional state of the fetal pulmonary vasculature. When interpreted together with other parameters, such as lung biometry, ductus venosus or umbilical artery Doppler, and clinical context, branch PA Doppler can provide valuable insights into pulmonary vascular resistance and right ventricular output. Its role is particularly growing in the context of lung maturity assessment and prediction of neonatal pulmonary outcomes, including risk of pulmonary hypoplasia and persistent pulmonary hypertension of the newborn (PPHN).
Imaging
- Axial Sweep (3-Vessel/3VT approach)
- Begin in the standard 4-chamber view.
- Sweep the probe cranially until the 3-vessel view (3VV)comes into plane (pulmonary artery, ascending aorta, and superior vena cava in cross-section).
- Continue a little further cranially into the 3-vessel-trachea (3VT) view — here, the main pulmonary artery (MPA) bifurcation into the right and left branch PAs can be seen clearly coursing laterally.
- Here, the bifurcation of MPA lies between the 3VV and 3VT views. Slow down the sweep between the two views to visualise RPA crossing from left to right.

- Short-Axis (RVOT Outflow) Sweep
- From the 4-chamber view, rotate/sweep cranially and slightly cranial-right to bring the right ventricular outflow tract (RVOT) into view.
- In this short-axis cut, the main pulmonary artery arises anteriorly from the RV, and by sweeping slightly, its division into branch PAs (leftward and rightward) is displayed.
- If we continue this view posteriorly, this will continue as a ductal arch. Alternatively, we can trace the branch PAs anteriorly from the ductal arch
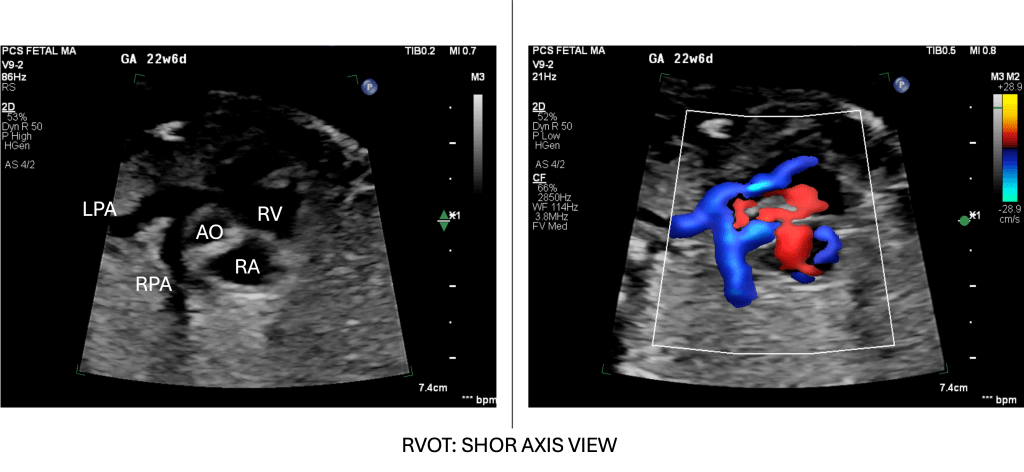
The branch PAs are usually best visualised in fetuses ≥20 weeks, and initial practice of delicate sweeps in good echo windows is recommended before attempting to visualise them in technically complex cases where echo windows are typically not optimal.
- Sample volume: Small (~1–2 mm), placed mid‐vessel in a branch PA.
- Angle of insonation: Like for all dopplers, aim for <20°, adjusting fetus or probe to align flow and beam
Key Doppler Parameters
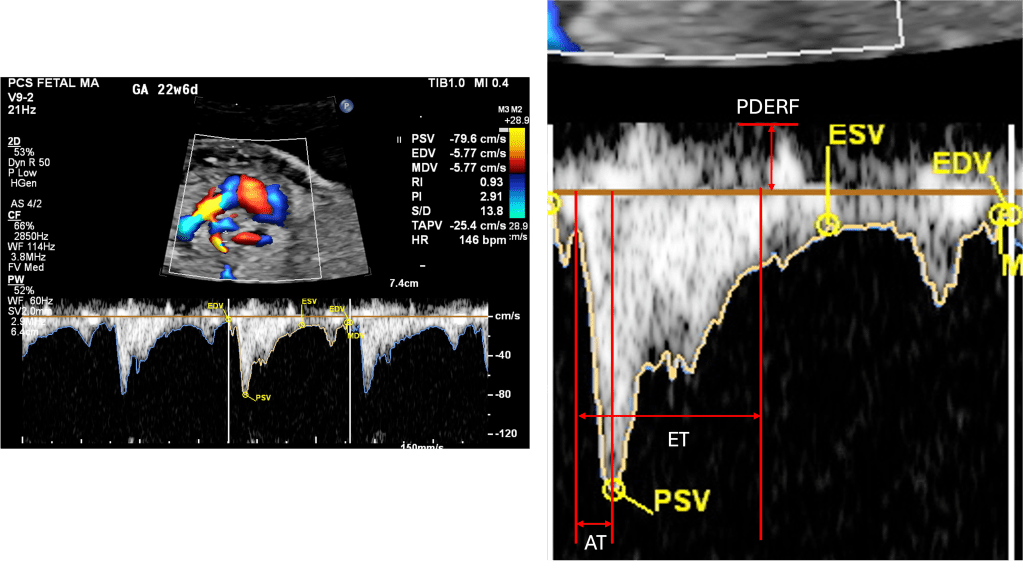
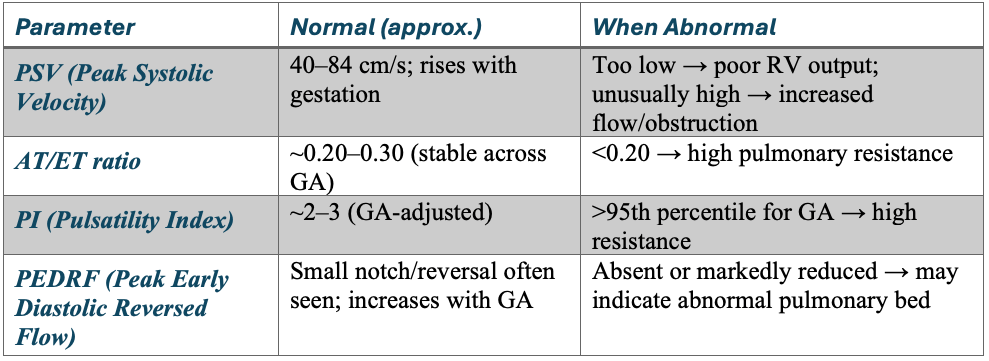
PSV – Peak Systolic Velocity
- The highest point of the systolic wave.
- Reflects the strength of proper ventricular systolic ejection into the branch PA.
- Lower PSV may be observed in severe right ventricular dysfunction or Ebstein anomaly; higher values may occur in states of increased flow.
AT – Acceleration Time
- Interval from the onset of forward flow to the peak systolic velocity.
- Sensitive to pulmonary vascular resistance (PVR).
- Short AT → high PVR (as in pulmonary hypoplasia, CDH, or restrictive atrial septum in HLHS).
- Prolonged AT → lower PVR, as lungs mature or with oxygen vasodilation.
ET – Ejection Time
- Duration of forward flow from onset to end of systolic ejection.
- Prolongs slightly with gestation; provides denominator for AT/ET ratio.
AT/ET Ratio
- A dimensionless index: AT ÷ ET.
- Relatively stable in normal fetuses (~0.20–0.30).
- Lower ratio → elevated PVR (poor pulmonary vascular compliance).
- Used in CHD, CDH, and PPHN prediction.
EDV – End-Diastolic Velocity
- Velocity of forward flow at the end of diastole.
- Usually slight but forward (positive).
- Absent or reversed EDV is abnormal, suggesting high downstream resistance.
PEDRF – Peak Early Diastolic Reversed Flow
- A brief negative deflection after systole (just below baseline).
- Commonly seen in fetuses, not pathologic if mild.
- Excessive or persistent reversal may suggest abnormal impedance in the distal pulmonary vasculature.
PI – Pulsatility Index
- Calculated as: PI = {PSV – EDV}/Mean velocity
- A measure of downstream resistance.
- High PI → high resistance (e.g., CDH, pulmonary hypoplasia).
- Low PI → lower resistance, as lungs mature or after maternal oxygen.
Why and when
Indications to attempt branch PA Doppler
- Complex CHD where pulmonary blood flow or pulmonary vascular resistance (PVR) matters (HLHS, TOF with PS, Ebstein, pulmonary atresia).
- Pulmonary hypoplasia risk (CDH, prolonged oligohydramnios, renal agenesis).
- Suspected fetal pulmonary vascular disease or to predict PPHN.
- Maternal hyperoxygenation testing.
- Research or serial monitoring when lung/PA function is a clinical question.
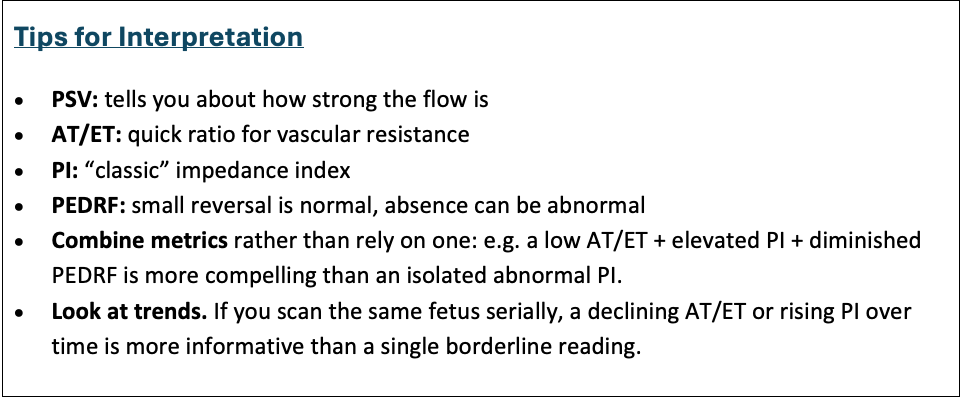
Interpretive rules
Normal branch PA
- Sharp systolic peak, small forward diastolic flow, AT/ET ≈ 0.20–0.30, PI within lab norms. → low-to-moderate fetal PVR for gestation.
High PVR / at-risk of PPHN / pulmonary hypoplasia
- Short AT, decreased AT/ET, high PI, absent or reversed EDV → high downstream resistance. Strongly consider CDH, pulmonary hypoplasia, HLHS with a restrictive atrial septum.
Low forward flow from RV (poor RV output)
- Low PSV, low amplitude waveform, absent diastolic flow → e.g., severe Ebstein, severe TR, pulmonary atresia physiology.
Proximal obstruction (e.g., TOF with PS)
- MPA waveform: increased proximal PI and altered shape. Distal branch PA may show reduced PI (dilated distal bed); compare branch vs MPA.
Parameter values and interpretation
TOF or Pulmonary Outflow Obstruction: Possible Role of Branch PA Doppler
In Tetralogy of Fallot (TOF) and related right ventricular outflow tract (RVOT) obstructions, most of the attention traditionally goes to proximal flow across the pulmonary valve or central PA. But branch PA Doppler can add unique downstream information:
What happens physiologically?
- In TOF with significant pulmonary stenosis or atresia, proximal resistance is high (pulmonary valve/MPA).
- The distal pulmonary vascular bed often adapts by becoming more vasodilated and low impedance.
- This means that, paradoxically, branch PA Doppler may show lower PI and more continuous forward flow, even though the proximal MPA Doppler shows high resistance.
What does this tell us?
- A low PI in the distal branch PA suggests that the lungs are well-adapted and capable of handling blood flow once RVOT obstruction is relieved.
- Conversely, suppose the branch PAs also show high PI, short AT, and absent/reversed diastolic flow. In that case, it raises concern that the distal bed is abnormal or poorly developed, which could complicate postnatal recovery.
How does this affect practice?
- Delivery planning: If branch PAs appear well-adapted (low PI, continuous forward diastolic flow), one can counsel parents that the lungs are likely ready to accept flow after surgical or catheter-based relief of obstruction.
- Timing of intervention: If branch PAs show poor adaptation, neonates may be at risk of early pulmonary hypertension or limited pulmonary flow even after RVOT is opened — prompting closer neonatal surveillance and perhaps earlier or staged intervention.
- Counselling: Adds depth to counselling parents beyond “there is TOF” → helps frame not just anatomy, but physiology.
Condition-Specific Pointers: Where Branch PA Doppler Changes Practice
While not part of routine fetal echocardiography, branch PA Doppler can directly influence management in high-stakes situations:
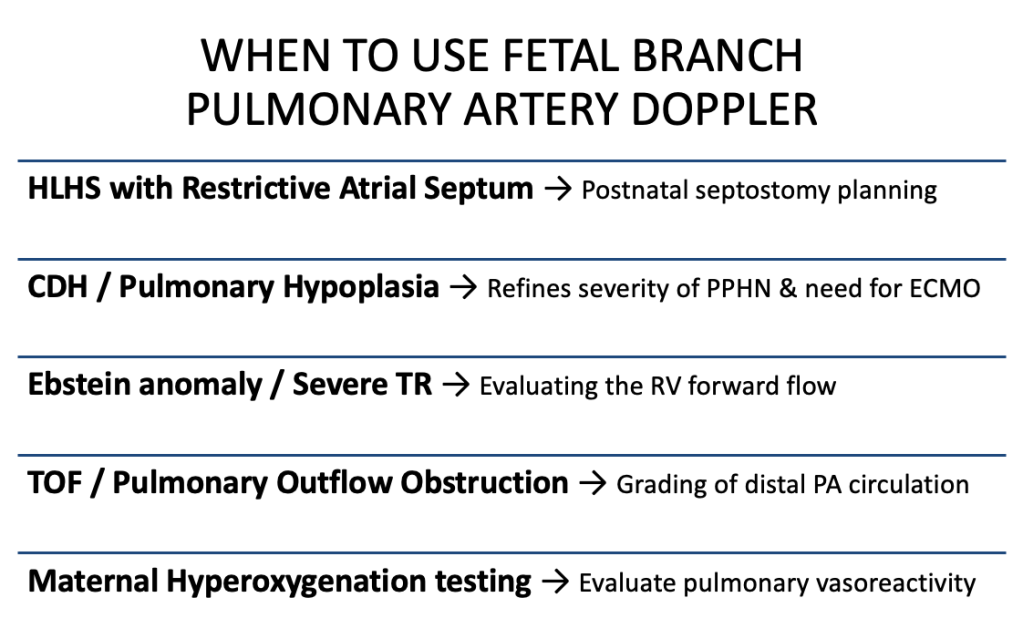
- HLHS with restrictive atrial septum → Short AT/ET, high PI, absent diastolic flow → strengthens the case for urgent postnatal atrial septostomy and justifies delivery at a tertiary cardiac centre.
- CDH / pulmonary hypoplasia → Elevated PI or absent diastolic flow, combined with lung size, improves prediction of severe PPHN and the potential need for ECMO → refines counselling and delivery planning.
- Ebstein anomaly / severe TR → Very low PSV and flat waveforms confirm severely reduced RV forward flow → supports the need for prostaglandin and specialised postnatal support.
- TOF / pulmonary outflow obstruction → Helps grade distal pulmonary vascular adaptation → may guide timing and intensity of neonatal intervention.
- Maternal hyperoxygenation testing → A fall in PI and rise in AT/ET = reactive pulmonary bed; no change = fixed high resistance → informs neonatal resuscitation and oxygenation strategy.
In these ways, branch PA Doppler does more than add numbers; it helps us stratify risk, plan delivery, and prepare both families and neonatal teams.
Conclusion:
Fetal branch pulmonary artery Doppler is not a routine practice, but in selected scenarios, it can be a practice-changing approach. When indices such as AT/ET ratio, PI, PSV, and PEDRF are integrated with anatomical findings and lung biometry, they provide functional insight into pulmonary vascular resistance and right ventricular output. This helps anticipate outcomes in HLHS, Ebstein anomaly, TOF, TAPVC, and CDH, and guides perinatal decision-making.
Looking ahead, its role is expanding — especially in fetal lung maturity assessment and maternal hyperoxygenation testing. These applications will be explored in forthcoming blogs, where we’ll discuss how branch PA Doppler can further refine counselling and delivery planning.

