Introduction & Prognosis
Ebstein anomaly is a rare congenital heart defect of the tricuspid valve, representing <1% of all congenital heart disease. Defined by apical displacement of the septal and posterior leaflets, it creates an atrialized portion of the right ventricle (RV). The clinical spectrum is broad, from mild cases with trivial regurgitation to severe presentations with hydrops and perinatal demise.
Prenatal diagnosis allows structured follow-up and delivery planning, but prognosis remains unpredictable. Some fetuses stabilise or even improve; others deteriorate with worsening cardiomegaly and circulatory failure.
Antenatal Physiology
The hemodynamic consequences of Ebstein anomaly stem primarily from tricuspid regurgitation (TR):
- RA enlargement and cardiomegaly
- Reduced RV forward output, sometimes functional pulmonary atresia
- Circular shunt physiology, where blood recirculate ineffectively between RA–RV–LV-PA without reaching systemic or Pulmonary circulation.circulation
- Venous congestion, seen as an abnormal ductus venosus or umbilical vein Doppler, indicating impending hydrops
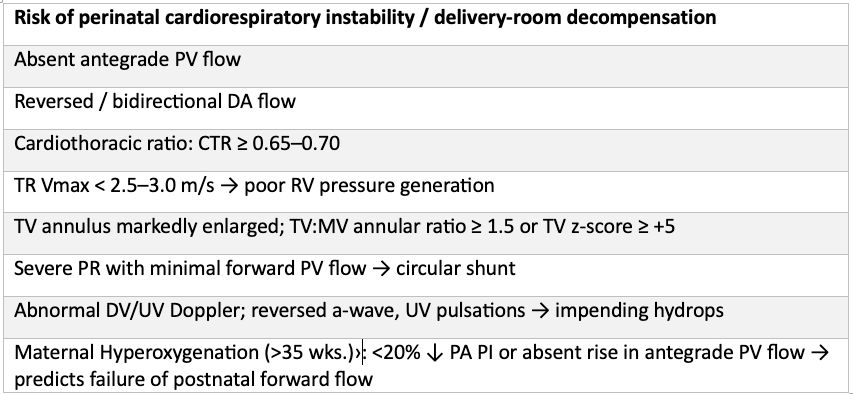
Risk Stratification
Early Pregnancy Counselling (<24 weeks)
At mid-gestation, the emphasis is on uncertainty.
- Adverse features: severe TR, cardiomegaly, reversed ductus venosus flow, pericardial effusion
- Associations: chromosomal and syndromic findings; maternal lithium exposure
- Genetic testing should be offered.
Parents must understand the broad spectrum of outcomes, ranging from termination to survival with surgical repair.
Near-term assessment
By late gestation, risk markers for perinatal instability should be actively sought.
Poor prognosis (demise/hydrops or extreme intervention)
- Hydrops fetalis (ascites/effusions/skin edema).
- Progressive cardiomegaly with CTR ≥ 0.70 despite expectant management
- Persistent circular-shunt physiology (reversed DA + absent antegrade PV) on serial studies
- Severely depressed RV function (qualitative or fractional shortening ↓) with TR Vmax low
Counselling at Term & Delivery Planning
- Delivery site: Ideally, it should be at a tertiary cardiac surgical centre.
- Preparedness: Prostaglandins, ventilation, inotropes, ECMO if needed
- Surgical options: Early palliation (Starnes procedure, systemic-to-pulmonary shunt) for unstable neonates; delayed repair possible in stable cases
Even reassuring prenatal findings cannot exclude the possibility of delivery-room decompensation; therefore, families should be prepared for this uncertainty.
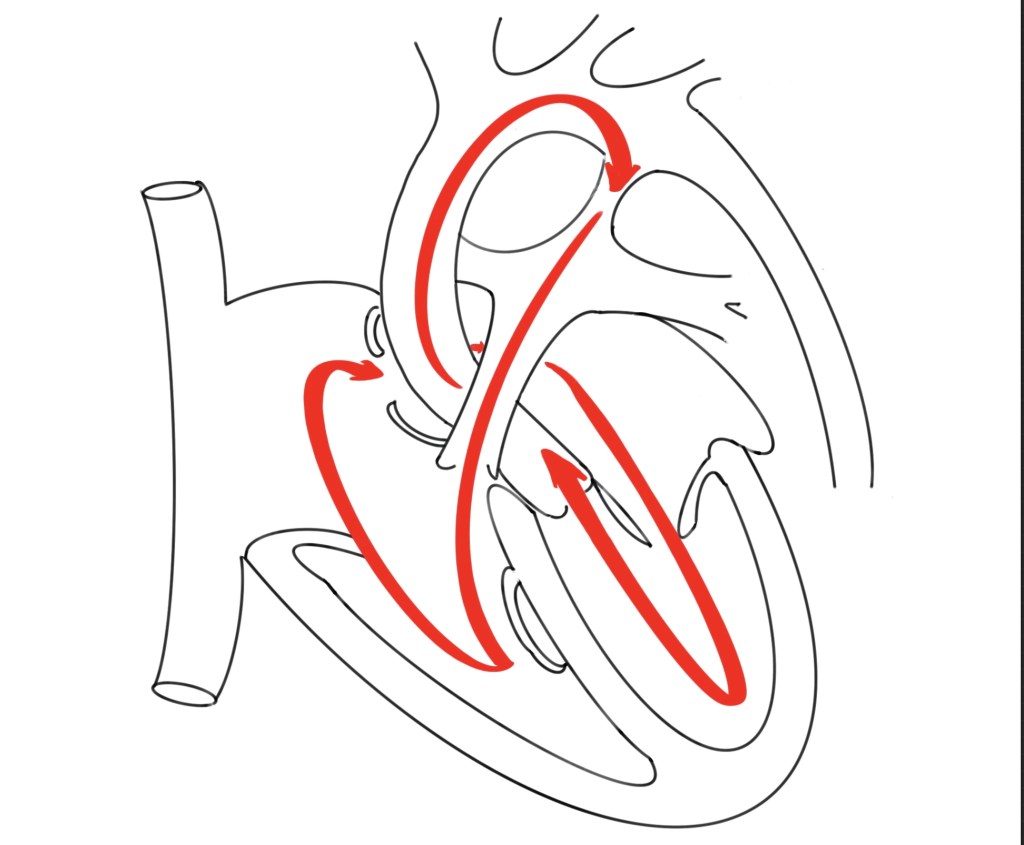
Circular Shunt: The Critical Mechanism
Circular shunt physiology is the hallmark of end-stage hemodynamic compromise (sometime called as “circle of death“) in severe Ebstein anomaly. It arises when two lesions coexist:
- Severe tricuspid regurgitation (TR) → large regurgitant volume from RV back into RA.
- Pulmonary valve incompetence → regurgitant pulmonary flow returning into the RV during diastole.
Together, these create a closed loop circuit:
LV→ AO → DA →PA → PR →RV → RA (Severe TR) →FO →LA →LV (again)
Hemodynamic Consequences
- Ineffective systemic output: Blood trapped in a right-sided loop contributes little or nothing to systemic circulation.
- RV energy wastage: The RV cycles blood through an ineffective loop, further exhausting the limited contractile reserve.
- Right atrial and venous congestion: Severe RA dilatation increases systemic venous pressures, reflected in reversed ductus venosus a-wave or umbilical venous pulsations.
- Pulmonary hypoplasia risk: Minimal effective antegrade pulmonary flow deprives lungs of normal distending shear stress, limiting alveolar development.
- Progression to hydrops: Inefficient circulation and venous hypertension trigger fluid accumulation and, ultimately, fetal demise.
Dynamic Evolution
- The Circular shunt is often not present in early mid-gestation; it develops later as TR worsens and pulmonary valve regurgitation appears.
- This underlines the importance of serial surveillance: a fetus stable at 20–24 weeks may evolve into a high-risk circular shunt physiology by the third trimester.
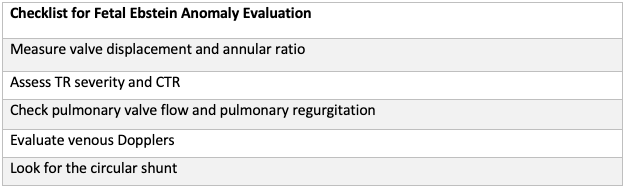
Fetal Ebstein Anomally Checklist
Conclusion: What the Risk Markers Represent
The constellation of fetal risk markers in Ebstein anomaly — absent pulmonary forward flow, reversed DA flow, severe TR with low jet velocity, marked annular dilatation, circular shunt physiology, and abnormal venous Dopplers — all point to the same final common pathway:
- Low RV contractile reserve → the RV is unable to generate pressure sufficient for effective antegrade ejection.
- Right-sided over-recirculation → blood is trapped in an inefficient loop, contributing little to systemic output.
- Ineffective pulmonary forward flow → both the lungs and the systemic circulation are underfilled, driving hypoxemia and pulmonary underdevelopment.
- Venous hypertension and preload compromise → abnormal DV/UV waveforms highlight the impact of impaired diastolic filling and systemic venous congestion.
In clinical terms, these markers are not just sonographic findings — they are surrogates of a failing right heart system in utero. Recognising them allows us to stratify fetuses into those who may transition safely at birth versus those who are at extreme risk of immediate collapse.
Key Take-Home
Fetal Ebstein anomaly should be viewed not only as a structural malformation but as a progressive hemodynamic disease. Identifying and integrating these markers into counselling and delivery planning is central to improving outcomes.

