Introduction: Why this matter?
Antenatal diagnosis of d-transposition of the great arteries (d-TGA) is one of the real successes of modern fetal cardiology. Survival after the neonatal arterial switch operation now exceeds 95% in experienced centres, and most children go on to need only one corrective procedure, with a low chance of re-intervention later in life.
For parents, this perspective is essential when discussing continuation of pregnancy and knowing that their baby’s outlook is excellent and the treatment is well established can make decision-making more transparent at a difficult time.
Still, the first hours after birth can be critical, especially if mixing at the atrial level is inadequate. This is why risk assessment near term matters: it does not change the fact that nearly all these babies survive, but it helps identify those who may need urgent stabilisation. Delivery at a tertiary cardiac centre is ideal, ensuring immediate access to specialised care. When families strongly prefer delivery elsewhere, antenatal echo markers can guide whether this is a reasonable plan, provided a clear and rapid transfer pathway is in place.
Antenatal physiology of FO and DA in dTGA
Understanding the antenatal hemodynamics of d-TGA explains why we focus on the foramen ovale (FO) and ductus arteriosus (DA):
• In d-TGA, the left ventricle ejects into the pulmonary artery (LV → PA). Pulmonary arterial oxygenation is relatively high, leading to a decrease in pulmonary vascular resistance and an increase in pulmonary blood flow.
• Increased pulmonary venous return raises left atrial (LA) pressure, which tends to limit right→left flow across the FO. FO restriction risks inadequate mixing after birth and may necessitate urgent balloon atrial septostomy (BAS).
• High oxygen content in the pulmonary/ductal circulation may also promote ductal constriction in late gestation; a restrictive DA further reduces mixing and can worsen pulmonary arterial pressures.

Early-trimester workup (18–24 weeks)
Goal at 18–24 weeks: confirm diagnosis, exclude additional lesions, and document morphologic risk markers that predict later FO restriction (which usually appears later).
Confirm
• Confirm d-TGA anatomy with intact septum or VSD.
• Look for associated lesions (VSD, outflow obstruction, aortic arch anomalies, small thymus), which change counselling and surgical planning and genetic association.
Risk Assessment
• Foramen ovale (FO): absolute diameter (mm), FO: AV ratio, flap mobility (normal vs tethered vs aneurysmal).
• Pulmonary veins: record S-wave (baseline numeric value).
• Ductus arteriosus (DA): calibre and basic Doppler.
Counselling after early diagnosis before 24 weeks
- Surgery & outcome: Almost all d-TGA babies require an arterial switch operation in the neonatal period. In high-volume centres, survival now exceeds 95%, and most children need only this single, definitive repair.
- Reintervention risk: Reinterventions are uncommon; however, factors such as coronary anatomy or arch anomalies may increase the complexity.
- Genetics: If there are associated anomalies, particularly aortic arch or thymic hypoplasia, discuss the possibility of 22q11 deletion and offer genetic counselling/testing.
- Parental choice (continuation vs discontinuation):
- Be transparent that outcomes are generally excellent with modern care.
- However, acknowledge that parents may weigh risks of open-heart surgery, the financial burden, and possible complications differently.
- Before 24 weeks, if parents consider termination, provide balanced, non-directive counselling: explain the medical facts, highlight the high survival rates, but respect their values, resources, and concerns.
- Emphasise that this is a treatable congenital heart disease, unlike some lesions where survival is poor.
- Document the discussion, and if parents continue, plan follow-up scans; if not, ensure access to appropriate counselling and support services.
Follow-up plan: Repeat fetal echocardiogram at 32–34 weeks and 36–38 weeks (more frequently if markers are abnormal).
Near-term assessment
This is the clinical pivot for delivery planning — measure these late (≥35–38 weeks) and act on them.
A. Foramen Ovale (FO)
- FO diameter within 3 weeks of delivery:
- Diameter ≤ 6.5 mm (within 3 weeks of delivery) [Buca et al.]
- Diameter ≤ 7 mm [Gottschalk 2023]
- FO: AV ratio ≤ 0.65–0.70 [O.Patey et al]
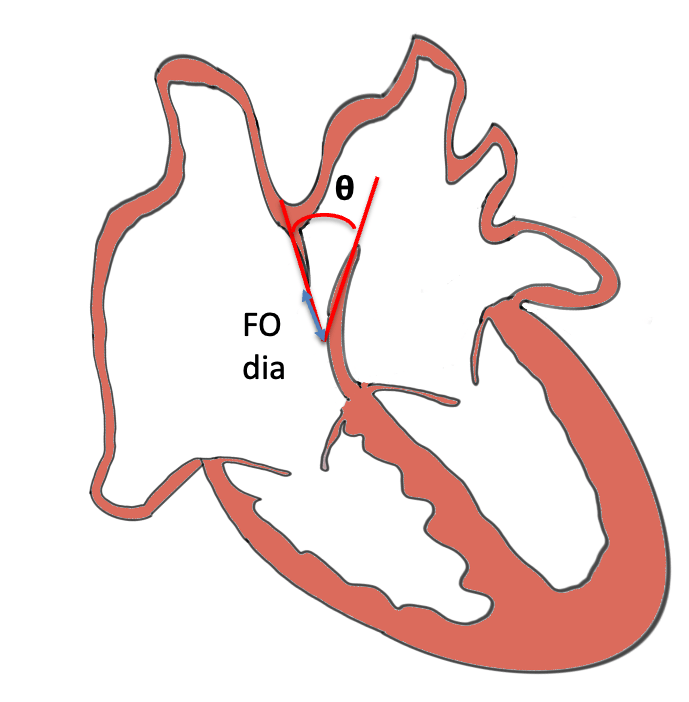
- FO Morphology / Mobility- Flap angle normally 30–50°; abnormal if <30° or >50° [Wilson et al.]
- Poor or absent FO flap mobility <30°
- Redundant- hypermobility /aneurysmal flap bulging (M-mode across LA-Septum-RA in Axial view) >50% across LA wall (‘spinnaker sail-like’) [Gottschalk 2023]
B. Pulmonary venous Doppler
- Pulmonary venous S-wave > 41 cm/s → marker of elevated LA pressure. [Słodki et al.]
C. Ductus arteriosus (DA)
- Ductal restriction features: peak systolic velocity > 1.4–1.5 m/s, diastolic velocity > 0.35–0.4 m/s, or DA z-score < −2.
E. Maternal hyperoxygenation (MH) as an adjunct (if available)
- Acute MH (100% O₂ for ~10 minutes) with pre/post Doppler can add information: failure of FO R→L shunting or <20% drop in branch PA PI suggests higher BAS need.
F. Do not over-rely on prenatal appearance:
“We should never trust fetuses with d-TGA, even when they seem to have a foramen ovale of normal appearance”. [Tuo et al.] Despite reassuring echo markers, some babies are “bad mixers” at birth and present with desaturation even with low antenatal risk factors.
Delivery Planning
- Site: tertiary cardiac centre for high-risk babies; otherwise, local delivery with transfer plan.
- Timing: aim for 39 weeks, daytime induction.
- Mode: as per obstetric indication.
- Immediate readiness: PGE1 available, intubation/ventilation equipment, NICU and cath team alerted.
Conclusion
Assess → Plan → Prepare.
In d-TGA, careful attention to the foramen ovale and ductus arteriosus provides insight into how the newborn may adapt to this condition. Some fetuses will mix blood adequately, others may struggle if the FO is restrictive or the DA constricts. Keeping this in mind helps guide delivery planning, choosing the right setting and being ready, without assuming every case will follow the same course.

