A soft marker is defined as a finding on an antenatal ultrasound that can be associated with an increased risk for a chromosomal abnormality, such as aneuploidy, but which may also be seen in normal fetal development.
These markers are not defects themselves and often have no clinical significance on their own, but their presence may prompt further evaluation or testing to assess the health and development of the fetus.
It’s important to note that while soft markers can indicate an increased risk of a chromosomal anomaly, they are not diagnostic. Soft markers are usually detected during the second-trimester anatomy scan, which is usually done at 18-20 weeks of pregnancy.
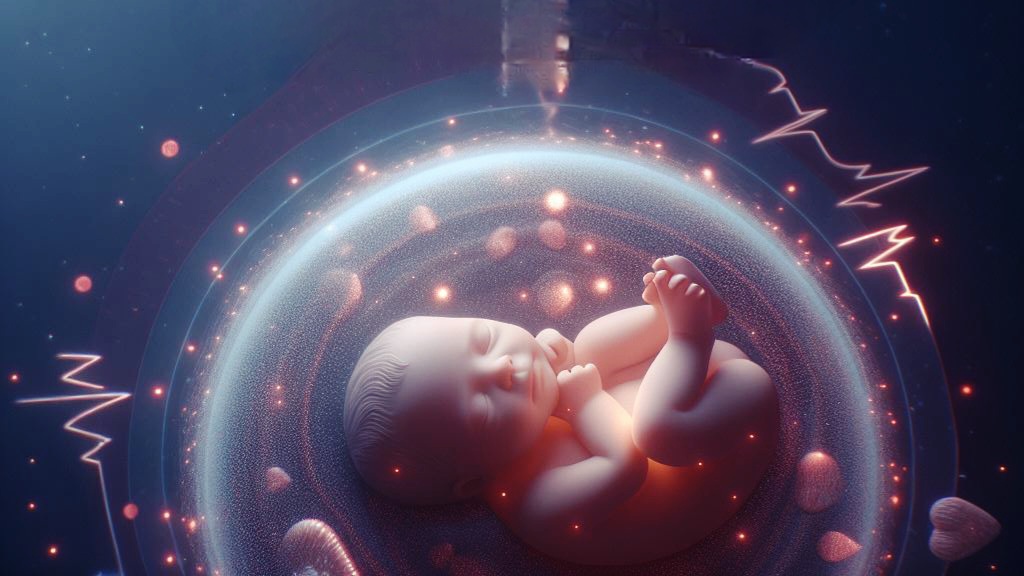
In the realm of fetal echocardiography, two significant soft markers often detected are the Echogenic Intracardiac Focus (EIF) and the Aberrant Right Subclavian Artery (ARSA). While ARSA is a structural abnormality, its association with trisomies also categorizes it as a soft marker. This dual nature highlights the complexity and importance of these markers in prenatal assessments.
Definition of Soft Markers
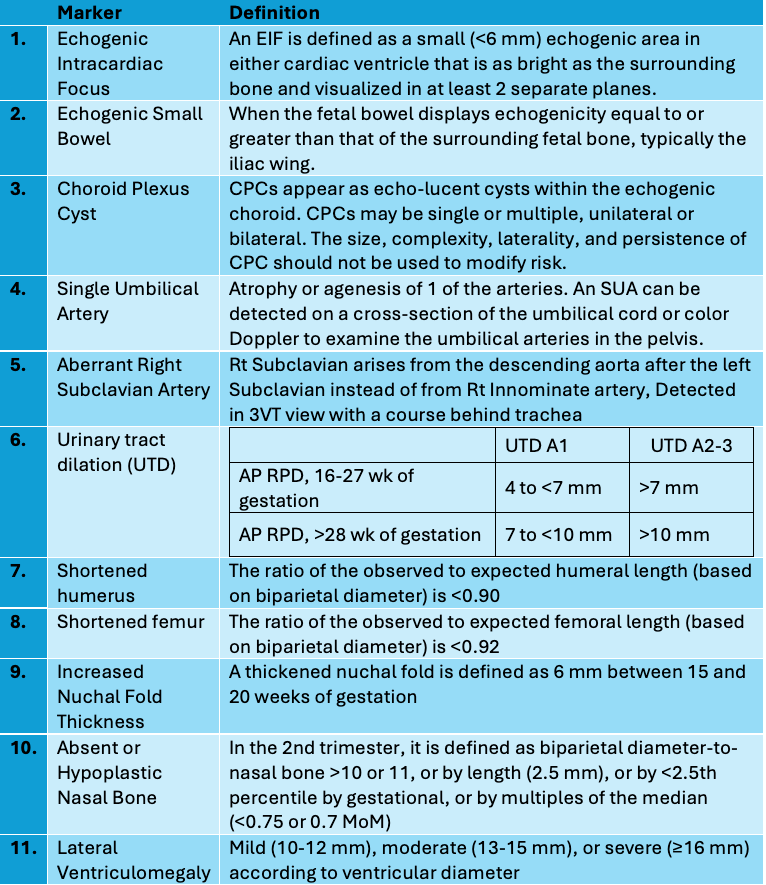
What steps are taken upon the identification of an isolated soft marker?
When an isolated soft marker is found during an ultrasound, the initial approach generally involves the following steps:
- Assessment of the Finding: Evaluate the soft marker within the context of the gestational age and the detailed anatomy of the fetus. It’s critical to determine whether the marker is truly isolated or if there are additional markers or anomalies.
- Review of Previous Screening: Examine the results of any previous aneuploidy screening tests, such as first-trimester screening, cfDNA testing, or quad screen. This information will help assess the individual risk and implications of the soft marker.
- Risk Communication and Counselling: Counsel the parents or patient about the significance of the soft marker. Explain the associated risks and reassure them that these markers are relatively common and often not associated with significant abnormalities in the presence of negative screening results.
- Discuss Options: If no screening has been done previously, discuss the options for further non-invasive screening tests or diagnostic procedures. This might include cfDNA testing or invasive methods such as amniocentesis, which can provide more definitive information.
- Shared Decision–Making: Engage the parents or patient in a shared decision-making process, taking into consideration their preferences, values, and any specific concerns they may have regarding the implications and next steps related to the finding.
- Follow-up: Depending on what was decided, arrange for additional testing or follow-up ultrasound exams to monitor the soft marker or assess fetal growth and well-being.
- Documentation: Document the findings, the discussions you’ve had, and the care plan going forward in the patient’s medical record.
Throughout this process, it’s important to provide clear, balanced information to support informed decision-making by the parents or patient and to ensure that any follow-up is appropriate based on the clinical circumstances and established medical guidelines.
When should genetic studies be considered upon the detection of an isolated soft marker?
The decision of whether and when to opt for genetic studies when an isolated soft marker is detected depends on a combination of factors:
- Previous Screening Results: If the patient has already had negative cfDNA or serum screening results, it may not be necessary to perform additional genetic studies for aneuploidy because the risk is considerably reduced.
- Baseline Aneuploidy Risk: Factors such as maternal age, family history, and other risk factors can influence the decision. Higher baseline risk may warrant additional testing
- Number and Type of Soft Markers: The presence of multiple soft markers or specific types of soft markers increases the risk of chromosomal abnormalities and suggests further genetic evaluation. The aggregated risk can be assessed with the help of LR calculations.
- Patient Preferences: Some parents might opt for further testing for more definitive answers, while others may decline due to the risks associated with invasive testing or personal beliefs.
- Clinical Circumstances: Aspects such as the health of the pregnancy, the presence of other anomalies, and overall fetal development can impact the timing and necessity for genetic studies.
- Availability of Tests: Certain tests may not be available in all areas, and cost may also be a consideration. Non-invasive prenatal testing can be a first step if invasive procedures are not immediately chosen.
- Gestational Age: The timing of the ultrasound finding might coincide with the clinical windows where certain tests are more informative or feasible.
Typically, genetic counselling is offered to discuss these factors in detail. The genetic counsellor can provide personalized risk assessment and guidance on the benefits and limitations of genetic testing options, which can include non-invasive prenatal testing (cfDNA), chorionic villus sampling, and amniocentesis. Parents can then make an informed decision that aligns with their needs and values.
Conclusion
Soft markers remain crucial in prenatal screenings for identifying fetal chromosomal abnormalities. These play a pivotal role alongside cell-free DNA (cfDNA) testing in risk assessment and management decisions. Detection of soft markers guides healthcare providers in stratifying pregnancies and determining the need for further testing, including cfDNA analysis. This comprehensive approach ensures timely follow-up and appropriate interventions, such as additional ultrasounds or advanced genetic testing, to monitor and clarify findings accurately.
